Which Best Describes a Beta Particle Answers.com
The electron beta - has a charge of. A certain radioactive element has a half-life of one hour.

Solved One Characteristic That Makes Alpha Radiation Chegg Com
DIt has a mass of 4 and a charge of 2.
. 11 H X 63 Li 42 He. The neutron beta particle and proton inside a neutron is a clear example of a negative particle beta particle and a positive particle proton experiencing electromagnetic force attraction between positive and negative charges at a very short distance. A 1e B -1e C 2He D Y check_circle Expert Answer.
2 25 points She Answer Phosphorous-32 is a beta emitter with a half-life of143 days. U atomic number 92 230. O A an electron emitted by an unstable nucleus OB a negatively charged particle that radiates X-rays and visible light C a positively charged particle with two protons and one neutron Da neutrino with no charge and no mass.
Check out a sample QA here. Neutron beta particle and proton last option in the list Explanation. A β particle is the antimatter equivalent of a proton.
Which best describes a beta particle. Which of these can be considered as the combination of a proton and an electron. Which best describes a beta particle.
A high-energy photon b. The concentration of carbon-14 in a body after 50000 years is too low. Which isotope is produced when osmium-191 emits a beta particle.
Which of the following symbols represents a beta particle. BIt has a mass of 0 and a charge of 1. Want to see this answer and more.
If you start with a 1-g sample of the element at noon how much of this same element will be left at 300 PM. Aalpha particle Bbeta particle. None of the answers are correct.
Polonium-210 decays into lead-206 and an alpha particle. Use a periodic table to answer the question. Uranium-235 and a neutron produce barium-141 krypton-92 and three neutrons.
The alpha particle has twice the electric charge of the beta particle but deflects less than the beta in a magnetic field because it _____. Carbon-14 breaks down into a beta particle and nitrogen-14. Which best describes a beta particle.
A It disappeared B It reacted with air. Group 2A Period 6. Which equation represents positron decay.
C It turned into sulfur and a beta particle. Which answer best describes an alpha particle. 11Which statement best describes gamma radiation.
A β particle is the antiparticle to the electron. Alpha particle beta particle gamma ray. The energy equation does not apply towards fission only fusion.
The three main types of nuclear radiation are alpha beta and gamma. CIt has a mass of 0 and a charge of 0. After 42 days a 100-mg sample.
A β particle is a high-energy electron. A β particle is another term for an electron orbiting a stable nucleus. 116 C 01 e 115 B.
The change of element into another by the process of nuclear decay is called. How many alpha particles are emitted in the series of radioactive decay events from a U-238 nucleus to a Pb-206 nucleus. A beta particle is released when a nucleus undergoes a beta decay process.
D It turned into silicon and a beta particle. Which of the following statements best describes the role of neutrons in the nucleus. Base your answer to the following question on the given the nuclear equation.
If uranium-234 decays via alpha emission what is the likely product of radioactive decay. As 14-C decays to 14-N the number of protons in the nucleus. Which statement BEST describes where the rest of the 32P went.
As a beta particle is released a neutron transforms into a proton. Dunstable nuclei emit beta particles 10Atoms of I-131 spontaneously decay when the AIt has a mass of 1 and a charge of 1. Which of these describes bariums location on the periodic table.
2 question 10. Which statement best describes the science of chemistry. In this process a neutron gets converted to a proton and an electron.
Which best describes a beta particle that is emitted during nuclear decay. The answer is a positively charged nucleus with two protons and two neutrons. Select the correct answer below.
A beta particle could be either a beta particle which is a positron or a beta - particle which is an electron. Does a radioactive isotope ever lose its radioactivity. A beta β particle is described by which of the following.
Experts are waiting 247 to provide step-by-step solutions in as fast as 30 minutes. When an atom loses an alpha particle its atomic number decreases by 2 units and its mass number decreases by 4 units. Want to see the step-by-step answer.
The electron released is known as beta particle only.

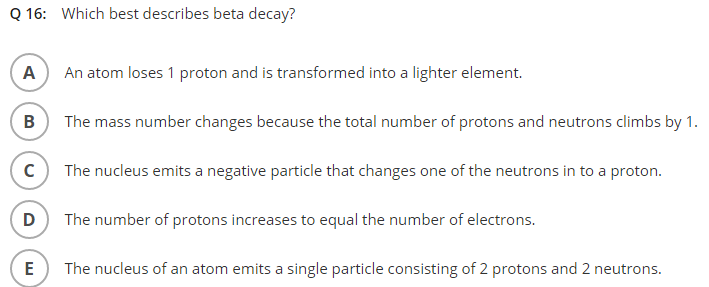
Solved Q16 Which Best Describes Beta Decay Aan Atom Loses Chegg Com

Solved Multiple Choice Choose The Best Answer To The Chegg Com

Question Video Identifying The Statement Which Best Describes Transuranium Elements Nagwa
0 Response to "Which Best Describes a Beta Particle Answers.com"
Post a Comment